BioPassport
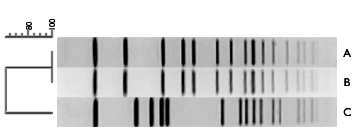
At MEI, we have devised a process called BioPassport, which characterizes, qualifies, and validates vaccine and production platform cell lines (prokaryotic and eukaryotic). The process involves: purity check, detailed RFLP analysis to establish distinguishing genetic fingerprints for the identification and tracking of each cell line and the stability of the unique cell line genetic determinants. Additional services also include viral or bacteriophage assays.
MEI addresses regulatory requirements for the testing of Master Cell Banks (MCB) and Working Cell Banks (WCB).
We provide the following analytical services in the characterization of microbial cell banks:
- Purity check by detection of contaminating microorganisms including bacteria and fungi-ensured through our highly accurate microbial identification system
- Identity of parental organism where application of our microbial identification system is critically applied in combination with genetic subtyping analysis to ensure species identity as well as strain stability
- Absence of contaminating bacteriophage, which is critical to guaranteeing stability of the microbial cell line during production
- PCR-based analysis of retention and stability of recombinant gene construct as well as examination of gene deletions and insertions critical for assigning identity and purity of platform and candidate strains
- Analysis of DNA sequence of gene of biological interest